Kala Pharmaceuticals, Inc. KALA announced that the FDA has accepted its investigational new drug (IND) application for its lead product candidate, KPI-012, which is being developed for the treatment of persistent corneal epithelial defect (PCED), a rare disease of impaired corneal healing.
The company plans to begin a phase IIb study evaluating KPI-012 for treating PCED in the first quarter of 2023.
The randomized, vehicle-controlled, parallel-group phase IIb study will enroll around 90 adult patients with PCED and is designed to evaluate the safety and efficacy of two doses of KPI-012 ophthalmic solution versus vehicle for 56 days. The primary endpoint of the study will check the complete healing of the PCED as measured by corneal fluorescein staining.
Top-line data from the above-mentioned study is expected in the first quarter of 2024.
Per the company, if data from the above-mentioned is found to be positive, this could serve as the first of two pivotal studies required for filing a biologics license application for KPI-012 to the FDA.
Shares of Kala Pharmaceuticals were up 33.4% in after-hours trading on Tuesday following the announcement of the news. The stock has plunged 93.3% in the past year compared with the industry’s decline of 22.8%.
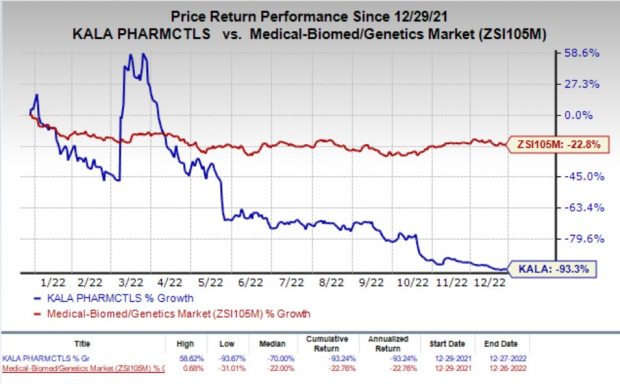
Image Source: Zacks Investment Research
In November 2022, the company submitted the IND application for KPI-012 to the FDA.
Per the terms of the private placement announced last month, Kala Pharmaceuticals sold an aggregate of 43,478 shares of Series E Convertible Non-Redeemable Preferred Stock at $575 per share to a life sciences focused-investor for $25 million following the IND acceptance by the regulatory body.
Kala Pharmaceuticals’ lead pipeline candidate, KPI-012 is a human mesenchymal stem cell secretome.
The FDA has already granted an orphan drug designation to KPI-012 for treating PCED. The company is also planning to develop KPI-012 targeting other ocular indications.
Successful development and potential approval for KPI-012 will be a huge boost for the company.
Zacks Rank & Other Stocks to Consider
Kala Pharmaceuticals currently carries a Zacks Rank #2 (Buy). Some other top-ranked stocks in the biotech sector are Syndax Pharmaceuticals, Inc. SNDX, Celularity Inc. CELU and Immunocore Holdings plc IMCR, all sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
Loss per share estimates for Syndax Pharmaceuticals have narrowed 5.9% for 2022 and 14.5% for 2023 in the past 60 days.
Earnings of Syndax Pharmaceuticals surpassed estimates in three of the trailing four quarters and met the same on the other occasion. SNDX witnessed an earnings surprise of 95.39% on average.
Loss per share estimates for Celularity have narrowed 57.1% for 2022 and 7.7% for 2023 in the past 60 days.
Earnings of Celularity surpassed estimates in three of the trailing four quarters and missed on the remaining occasion. CELU witnessed an earnings surprise of 51.01% on average.
Loss per share estimates for Immunocore have narrowed 56.1% for 2022 and 56.8% for 2023 in the past 60 days.
Earnings of Immunocore surpassed estimates in three of the trailing four quarters and missed on the remaining occasion. IMCR witnessed an earnings surprise of 68.34% on average.
Zacks Top 10 Stocks for 2023
In addition to the investment ideas discussed above, would you like to know about our 10 top picks for the entirety of 2023? From inception in 2012 through November, the Zacks Top 10 Stocks portfolio has tripled the market, gaining an impressive +884.5% versus the S&P 500’s +287.4%.
Now our Director of Research is combing through 4,000 companies covered by the Zacks Rank to handpick the best 10 tickers to buy and hold. Don’t miss your chance to get in on these stocks when they’re released on January 3.





