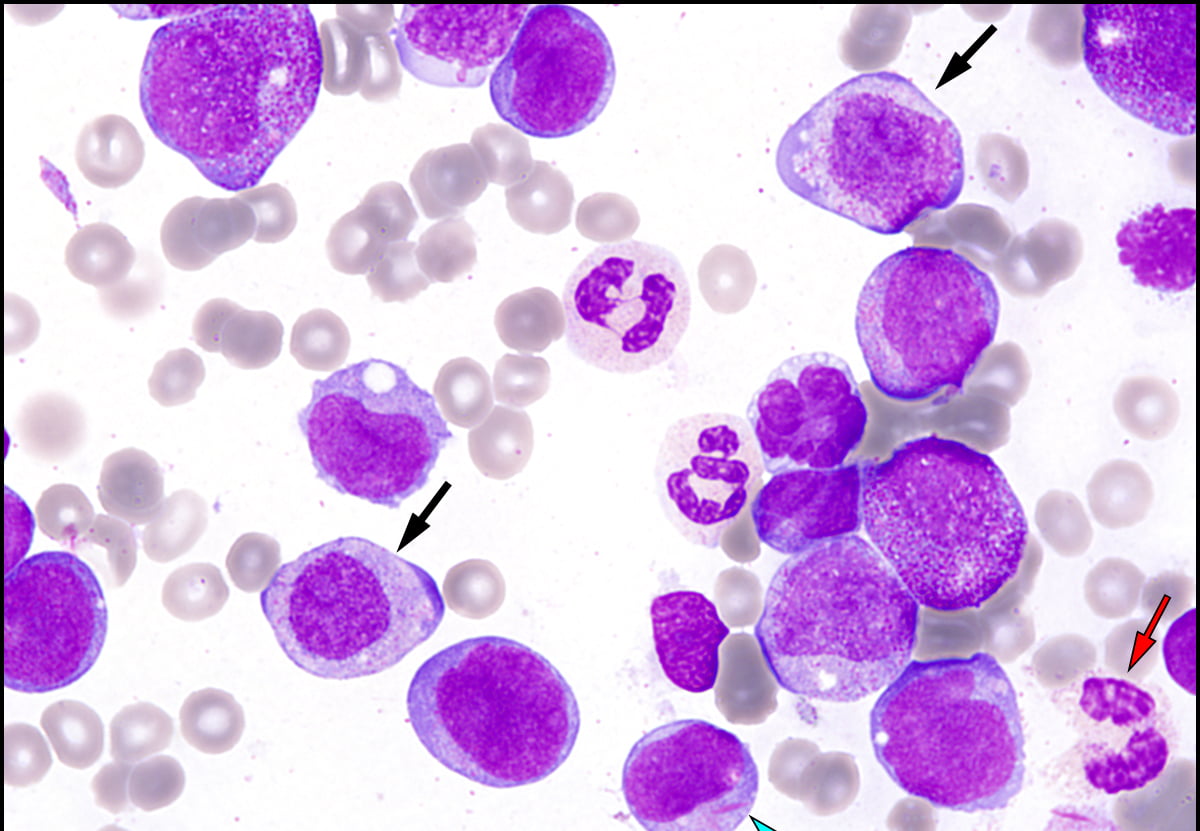
Basel, 19 October 2020 – Roche (SIX: RO, ROG; OTCQX: RHHBY) today announced that the US Food and Drug Administration (FDA) has granted full approval of Venclexta® (venetoclax) in combination with azacitidine, or decitabine, or low-dose cytarabine (LDAC) for the treatment of newly diagnosed acute myeloid leukaemia (AML) in adults 75 years or older, or who have comorbidities that preclude use of intensive induction chemotherapy. Venclexta was previously granted provisional approval in this setting under the FDA’s accelerated approval programme in November 2018. Today’s FDA approval converts Venclexta’s accelerated approval in this setting to a full approval.
“Today’s full approval is supported by the significant results that showed that Venclexta in combination with azacitidine extended overall survival for people with newly diagnosed acute myeloid leukaemia who cannot tolerate intensive induction chemotherapy,” said Levi Garraway, M.D., Ph.D., Roche’s Chief Medical Officer and Head of Global Product Development. “We are very pleased that this application was reviewed under the FDA’s Real-Time Oncology Review pilot and Project Orbis initiative, helping to bring this treatment option more rapidly to patients in the United States and other countries.”
The approval is primarily based on the results of two phase III studies, VIALE-A and VIALE-C. Results of the VIALE-A study, which were published in the New England Journal of Medicine in August 2020, showed Venclexta plus azacitidine significantly reduced the risk of death by 34% (overall survival; OS) compared to azacitidine alone (median OS=14.7 months vs. 9.6 months; HR=0.66; 95% CI: 0.52-0.85; p<0.001). People treated with Venclexta plus azacitidine had significantly higher rates of complete remission (CR) with 37% (95% CI: 31-43) compared to 18% (95% CI: 12-25) in people treated with azacitidine alone (p<0.001). The Venclexta plus azacitidine combination also led to higher rates of CR and CR with partial haematologic recovery (CR + CRh), with the combination showing a CR + CRh of 65% compared to 23% with azacitidine alone (p<0.001). The most frequent serious adverse reactions (≥5%), reported in 83% of people treated with Venclexta plus azacitidine, were low white blood cell count with fever (30%), pneumonia (22%), blood infection (excluding fungal;19%), and bleeding (6%).
For the VIALE-C study, the approval was based on the rate and duration of CR. Twenty-seven percent (95% CI: 20-35) of people treated with Venclexta plus LDAC achieved a CR (median duration of CR (DOCR)=11.1 months) vs. 7.4% (95% CI: 2.4-16) of people treated with LDAC alone (median DOCR=8.3 months). The median OS for people treated with Venclexta plus LDAC was 7.2 months vs. 4.1 months (HR=0.75; 95% CI: 0.52-1.07; p=0.114) for people treated with LDAC alone. These OS results were not statistically significant. The most frequent serious adverse reactions (≥10%), reported in 65% of people treated with Venclexta plus LDAC, were pneumonia (17%), low white blood cell count with fever (16%), and blood infection (excluding fungal;12%).
Updated results from additional phase I/II studies of Venclexta in people with newly diagnosed AML were included in the FDA submissions as supporting data.
“The results of the VIALE-A study reinforce the clinically meaningful benefit of Venclexta plus azacitidine for people newly diagnosed with AML,” said Courtney DiNardo, M.D., Associate Professor of the Department of Leukemia, Division of Cancer Medicine at The University of Texas MD Anderson Cancer Center. “Based on the results of this study, this treatment regimen represents a significant advance for older AML patients, including higher response rates, greater transfusion independence, longer durations of remission, and ultimately significantly improved overall survival compared to azacitidine alone.”
This is the second time that Venclexta has been reviewed under the FDA’s new Real-Time Oncology Review (RTOR) and Assessment Aid pilot programmes. The RTOR pilot programme explores a more efficient review process to ensure safe and effective treatments are available to patients as early as possible. The approval was also granted under the FDA’s recently established Project Orbis, which provides a framework for concurrent submission and review of oncology medicines among multiple regulatory agencies worldwide. Simultaneous applications were submitted to regulators in the US, Australia, Canada, Brazil and Switzerland under Project Orbis. Additionally, the FDA has granted five Breakthrough Therapy Designations for Venclexta, two of which are for people with previously untreated AML ineligible for intensive chemotherapy.
Venclexta is being developed by AbbVie and Roche. It is jointly commercialised by AbbVie and Genentech, a member of the Roche Group, in the US, and commercialised by AbbVie, under the brand name Venclyxto® outside of the US.