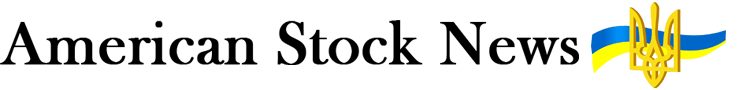EU financing for COVID-19 vaccine helps German firm close in on a solution with highly effective trial results
“It was oolong tea to be precise,” Türeci says. “We both like it and it’s easy to make. We didn’t have a lot time for a celebration.”
The two immune engineers are conscious of the vital nature of their research into a vaccine for the deadly disease that has devastated the entire globe in 2020. Both children of Turkish immigrants, Türeci, 53, and Şahin, 55, have led Mainz-based BioNTech to the frontrunner’s spot in the race for a vaccine. On 9 November, they announced that their vaccine, BNT162, showed indications of at least 90% efficacy in preventing COVID-19 infections, based on an interim analysis of the phase 3 trial.
The European Investment Bank is proud to have played a role in this success, signing two transactions with the company since 2019. The latest deal with BioNTech started early in the pandemic, when the Bank’s staff reviewed its portfolio and came up with BioNTech as one of the companies capable of developing a COVID-19 vaccine.
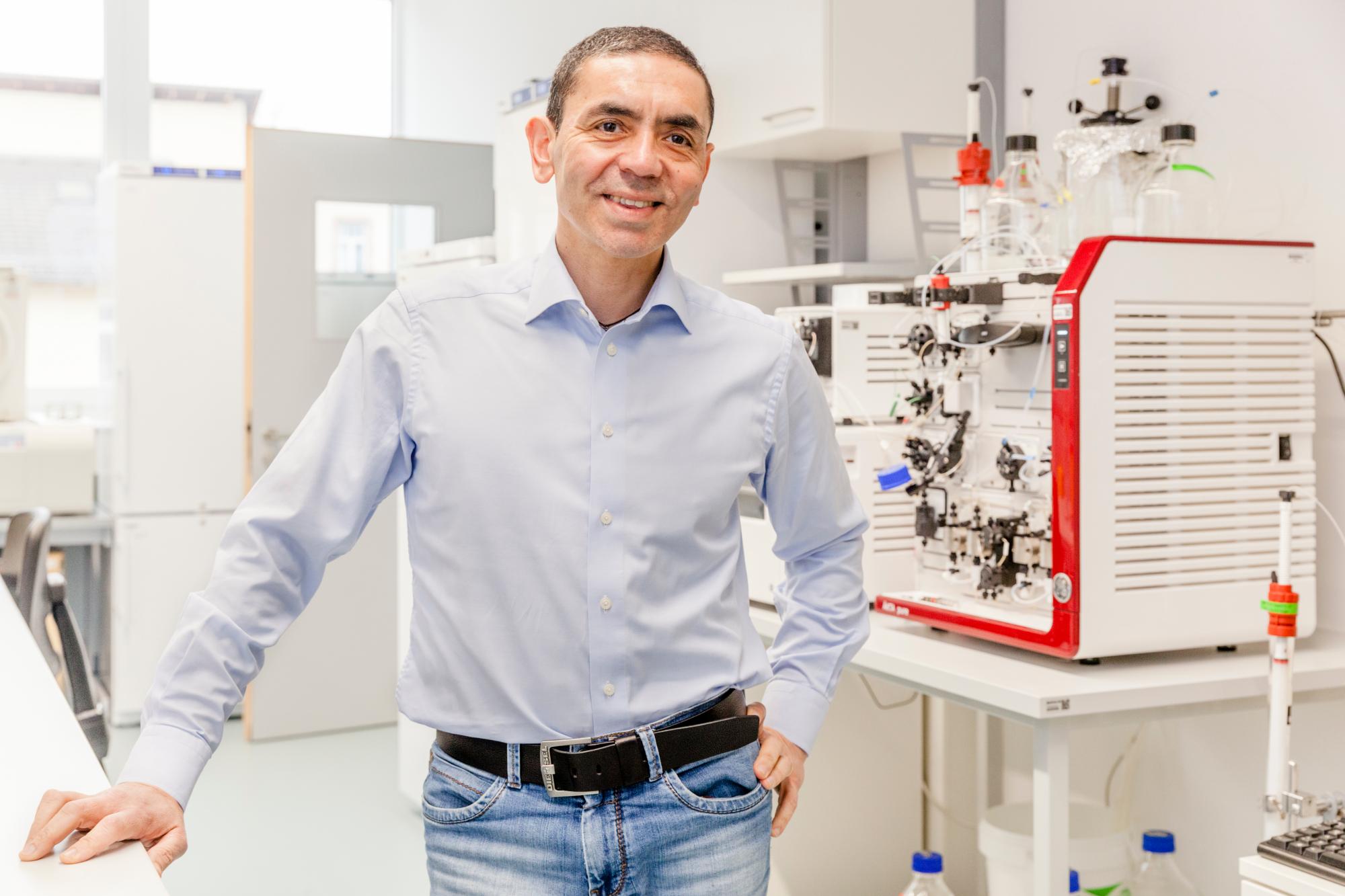
EU financing for COVID-19 vaccine backs innovative and risky projects
The EU bank had signed a €50 million loan in December 2019 to help the company work on cancer treatments and was impressed by the company’s team. The Bank signed the latest loan, worth €100 million, in June, after an accelerated approval that concentrated into two months a process that normally takes longer than a year. The new loan was designed to help BioNTech’s vaccine trials and manufacturing. The loan is backed by the InnovFin Corporate Research Equity programme and the European Fund for Strategic Investments, which support innovative and higher risk projects financed by the European Investment Bank with a guarantee from the EU budget.
“It really doesn’t get any better than this,” says Gergely Krajcsi, an investment officer at the European Invesment Bank who worked on the BioNTech deal. “The most we can contribute to the fight against COVID is to help companies, which are developing new vaccines, treatments or diagnostic solutions. We did everything we could to help make this vaccine happen.”
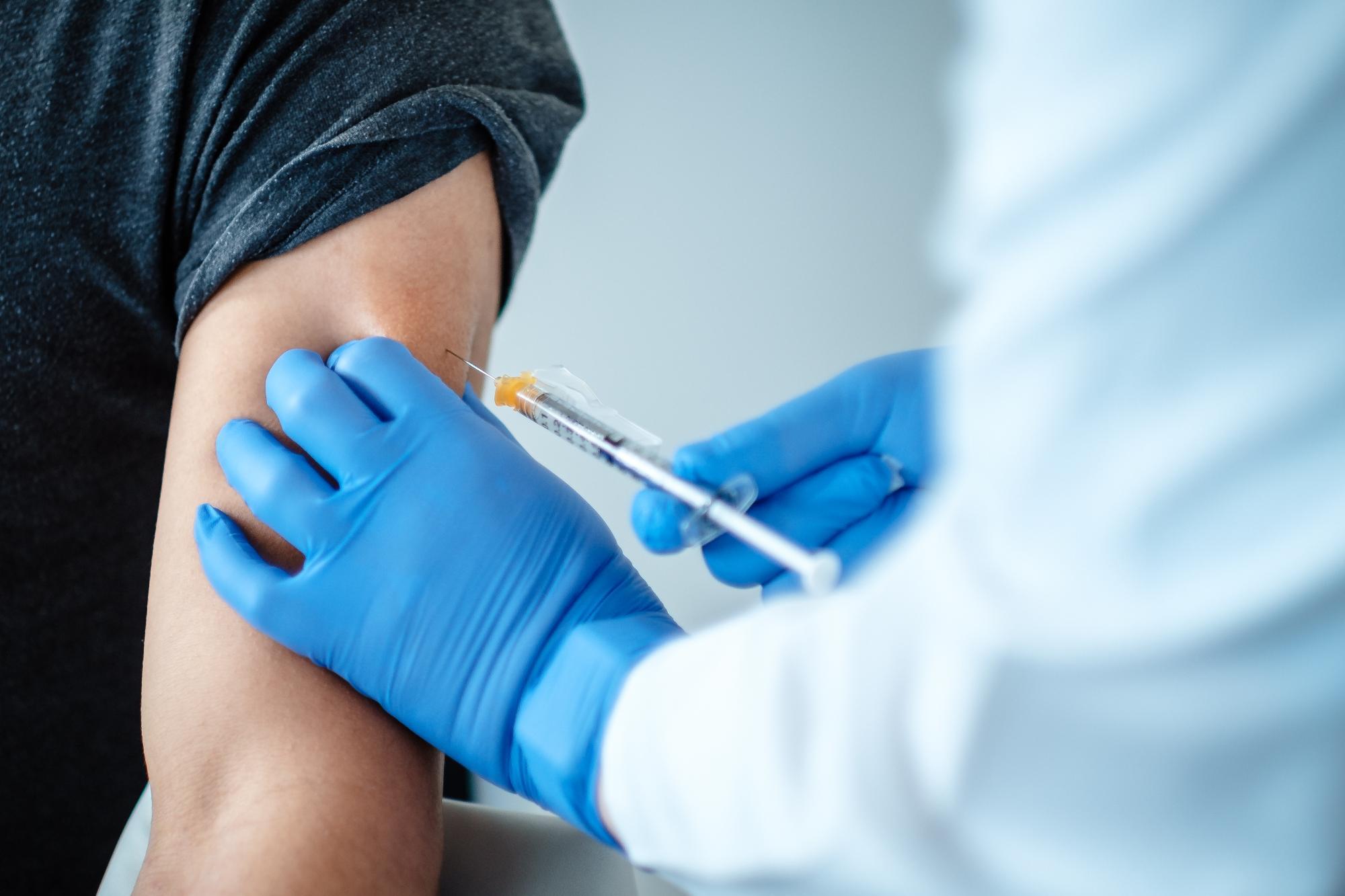
Advancing to the later stages in science
The BioNTech financing is one example among dozens that show how venture debt from a public bank is important in helping companies in the infectious disease sector get to the later stages of development, says Cristina Niculescu, a healthcare expert at the European Investment Bank. The private sector is reluctant to invest in this part of the economy, because companies are often startups or have little track record and the eventual success of any innovation is hard to predict.
“BioNTech is really exciting,” Niculescu says, “because it’s a European biotech company with great science that is now one of the vaccine frontrunners, and it’s a company that we already supported in the past.”
BioNTech also has a good story to tell about immigration and gender equality. A large percentage of the staff at the company are women. The parents of Türeci and of Şahin moved to Germany from Turkey in the late 1960s. “Immigrants are making big contributions here in Germany and across Europe,” says Türeci, who is the company’s chief medical officer. “It would be hard to do the research we’re doing without immigration.”
Over the past two decades, the couple have built a reputation as workaholics devoted to scientific research. On their wedding day in 2002, they started the morning by putting on lab coats and working a few hours in the office. They also have a laid-back style that encourages devotion from their nearly 1 500 employees. Türeci and Şahin, who is BioNTech’s chief executive officer, don’t own a car, and Şahin is known for riding his bike to work and entering meetings wearing jeans and holding a bicycle helmet.
The road to the COVID-19 vaccine
The husband and wife team co-founded BioNTech in 2008 to develop treatments for cancer. They wanted to run their own company to develop new therapies and innovations faster. Over the last decade, their company’s success has made the couple role models for scientists who also want to be entrepreneurs. BioNTech shifted its research to the COVID-19 vaccine in January. It called its COVID-19 project Lightspeed and told its hundreds of scientists that this would be a tough year.
Many employees cancelled ski holidays and the company started running day and night shifts, as well as staying open on weekends, to speed up a project that normally takes many years to complete. BioNTech is working with the pharmaceutical company Pfizer to expand its expertise for drug trials and the vaccine’s distribution.
BioNTech’s vaccine is different from conventional ones made for influenza or measles, which use an inactive or weakened form of the virus as a treatment. BioNTech, and several other companies, are using a technology that takes a small, non-infectious piece of genetic information called messenger RNA and injects it into muscles. This makes the body’s cells produce a protein resembling the spikes on the new coronavirus. This new protein prompts the immune system to make antibodies that prevent the disease. BioNTech’s proposed treatment requires two shots to be effective.
The successful early analysis of BioNTech’s trials puts the company’s vaccine safety and efficacy on par with other highly effective vaccines for diseases such as measles. But Türeci recognises there’s a lot of hard work ahead before she and her husband will have time for another cup of tea.
“Now we have to see how long the immunity lasts,” Türeci says. “We have ticked the first box, but there are more boxes we still have to tick.”