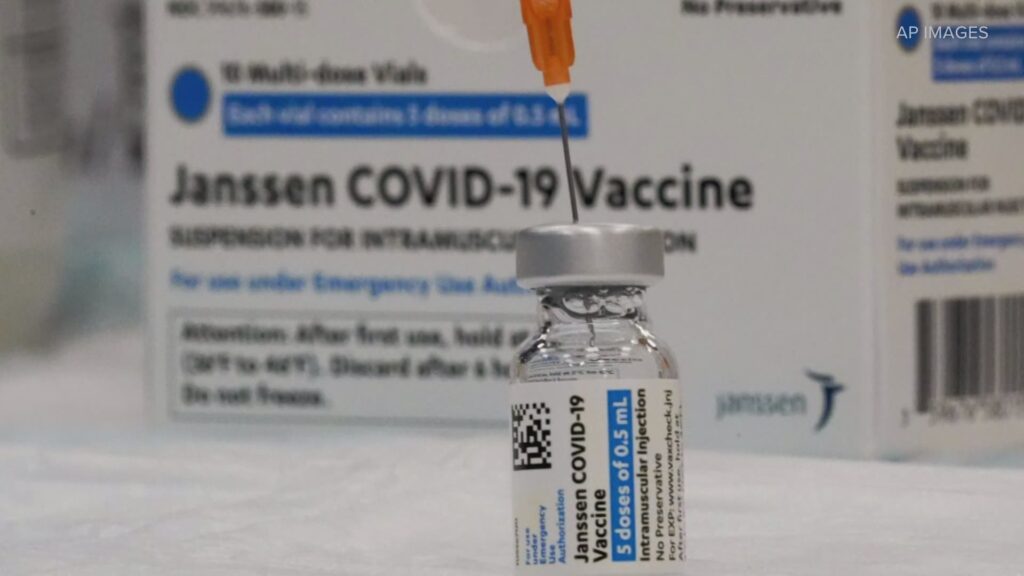
The Food and Drug Administration and the Centers for Disease Control and Prevention have recommended a pause in the distribution of the Johnson & Johnson COVID-19 vaccine after six women between the ages of 18 and 48 experienced clotting six to 13 days after vaccination.
MoreThe Food and Drug Administration recently granted emergency use authorization to a coronavirus vaccine from Pfizer, and approval for Moderna’s version is anticipated shortly.
More