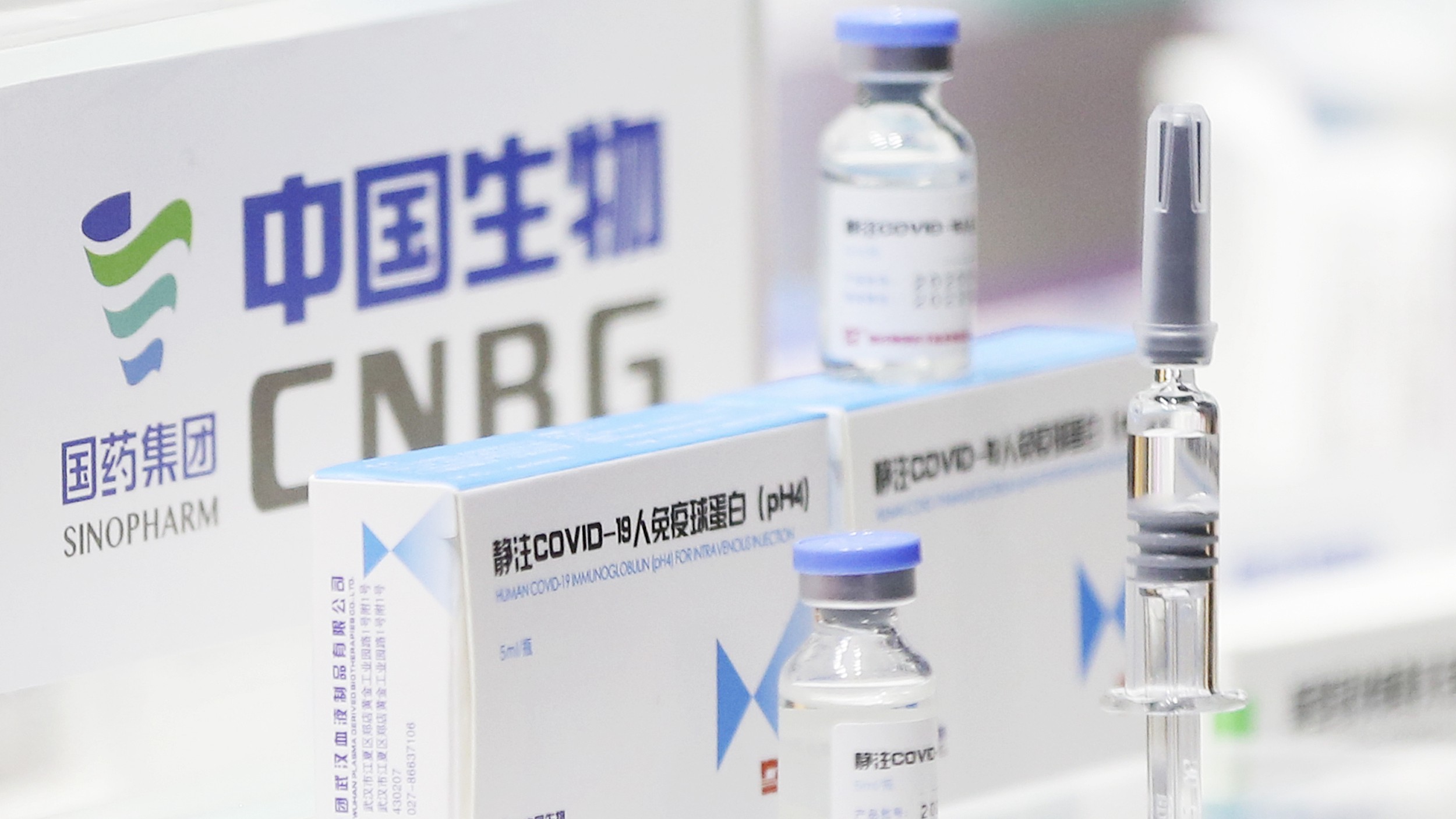
The government’s National Medical Products Administration announced Thursday that it had given conditional approval to a vaccine developed by Beijing Biological Products Institute, a subsidiary of state-owned Sinopharm. The regulatory agency granted the approval a day after Sinopharm said the vaccine had an efficacy rate of 79.3% against the coronavirus in a final large-scale clinical trial.
However, outside experts have questioned Sinopharm’s claims, since it has not provided the necessary data so it can be independently verified.
The newly approved vaccine is one of five developed by Chinese companies that have already been administered under its emergency use program while still undergoing Phase 3 trials. More than 4.5 million doses have been administered since July to essential workers and people considered high-risk, including 3 million since mid-December. Beijing is aiming to vaccinate as many as 50 million people by mid-February, when hundreds of millions of Chinese citizens are expected to travel for the annual Lunar New Year holiday.
China has pledged to provide hundreds of millions of doses of its homegrown vaccines to many developing nations around the world to promote global public good. Observers say the pledge is Beijing’s attempt to repair its image after failing to inform the world about the true nature of the virus, which was first detected in the central city of Wuhan one year ago.
Other vaccines
The United Arab Emirates granted emergency use approval for a Sinopharm-developed vaccine earlier this month after it was shown to be 86% effective in preventing moderate and severe cases of the virus in a late-stage clinical trial in September.
The Sinopharm vaccine joins other potential coronavirus vaccines to receive approval of governments around the world. Britain’s medical regulatory agency announced Wednesday that it has granted emergency authorization of a coronavirus vaccine developed jointly by British-Swedish pharmaceutical giant AstraZeneca and Oxford University.
Late-stage clinical trials of the AstraZeneca-Oxford vaccine revealed it to be 70% effective against COVID-19. The vaccine had a 62% efficacy rate for participants given a full two doses, but tests of a smaller sub-group revealed it to be 90% effective when given a half-dose followed by a full dose weeks later.
The AstraZeneca/Oxford vaccine is the second to be approved by Britain for its mass inoculation effort, which began earlier this month with the vaccine developed by U.S.-based Pfizer and Germany’s BioNTech. The new vaccine will be distributed across the country within days, with Britain having already ordered 100 million doses.
Unlike the Pfizer-BioNTech vaccine, which must be stored in super-cold refrigerators at temperatures below 70 degrees Celsius, the newly approved vaccine can be stored at normal temperatures of 2 to 8 degrees Celsius, making it easier to transport and administer to people in poorer and remote nations.
However, the AstraZeneca-Oxford vaccine has come under intense scrutiny over the number of people who took part in the smaller subgroup, which was just 2,741, and whether it is effective for people over age 55.
The new vaccines are coming as a more contagious strain of COVID-19 first detected several days ago in Britain has been identified at various points on the globe. Officials in California on Wednesday announced the variant has surfaced in the southern city of San Diego. The neighboring state of Colorado was the first in the United States to report the new strain earlier this week.
A different variant of the coronavirus has been detected in South Africa.