Kala Pharmaceuticals, Inc. KALA announced that the FDA has accepted its investigational new drug (IND) application for its lead product candidate, KPI-012, which is being developed for the treatment of persistent corneal epithelial defect (PCED), a rare disease of impaired corneal healing.
MoreMillions of people in the United States have received COVID-19 vaccines under the most intense safety monitoring in U.S. history
MoreU.S. pharmaceutical company Pfizer announced Friday its new COVID-19 pill showed an 89% reduction in risk of COVID-19-related hospitalization or death in clinical trials and they plan to submit the drug to U.S. regulators for emergency use approval.
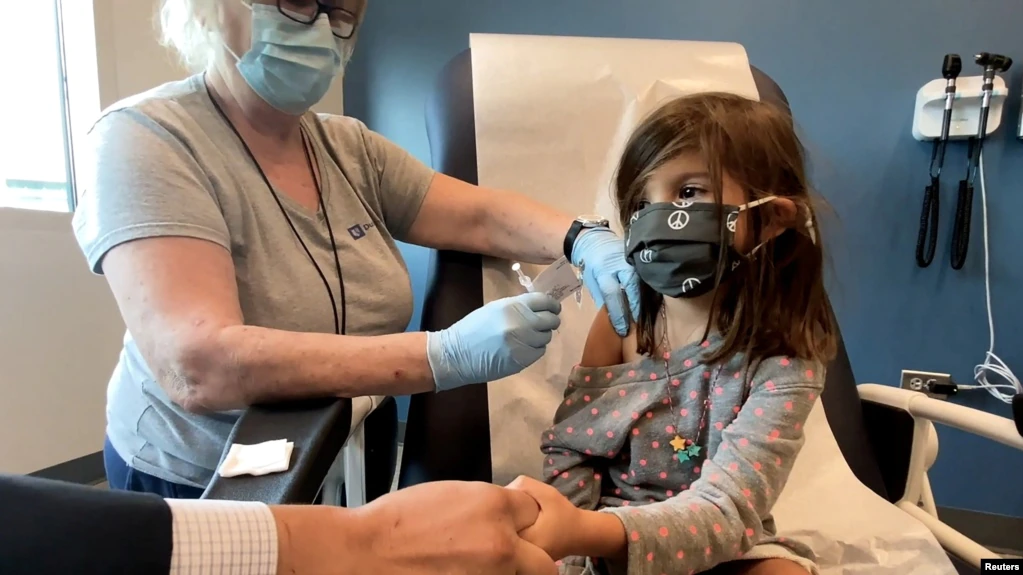
MoreThe U.S. Food and Drug Administration's independent advisory committee is meeting Tuesday to consider giving emergency approval to the Pfizer-BioNTech COVID-19 vaccine for children ages 5 to 11.
MoreThe U.S Food and Drug Administration's (FDA) vaccine advisory panel is meeting Friday to consider drug partner Pfizer-BioNTech's application to offer their COVID-19 vaccine as a third-dose booster for patients 16 and older.
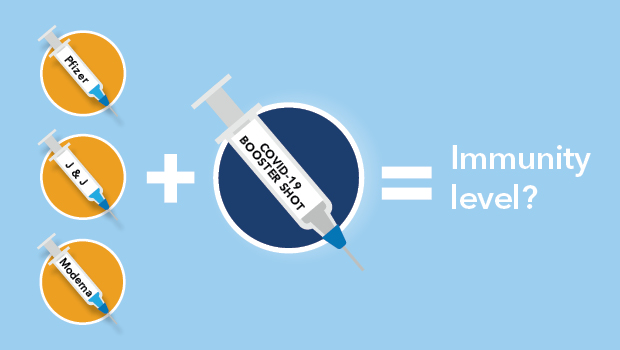
MoreThe committee that advises the FDA on vaccines will meet on Friday to discuss booster shots for all Americans above the age of 16.
MoreRoyal Philips (NYSE: PHG; AEX: PHIA) today announced an update in connection with the June 14, 2021 recall notification* for specific Philips sleep and respiratory care devices that was issued to address potential health risks related to the polyester-based polyurethane (PE-PUR) sound abatement foam component in these devices.
MoreNOTICE: CDC continues to recommend the use of the newly FDA-approved Pfizer-BioNTech (COMIRNATY) COVID-19 Vaccine for people 16 years and older, as one of the recommended vaccines to protect against COVID-19.
MoreImportant information for healthcare providers and the public included in updated FDA Emergency Use Authorization Fact Sheets
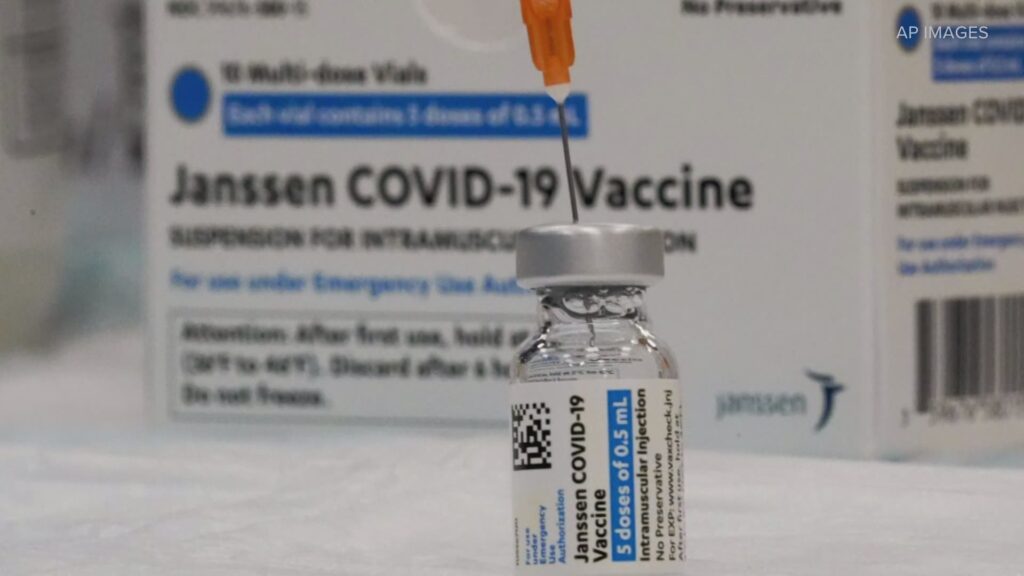
MoreThe Food and Drug Administration and the Centers for Disease Control and Prevention have recommended a pause in the distribution of the Johnson & Johnson COVID-19 vaccine after six women between the ages of 18 and 48 experienced clotting six to 13 days after vaccination.
More