Rapid test to support the American public's fight against the COVID-19 pandemic, with availability to purchase over-the-counter (OTC) at pharmacies and retailers nationwide
MoreRoche (SIX: RO, ROG; OTCQX: RHHBY) and GenMark Diagnostics, Inc. today announced that Roche's wholly owned subsidiary Geronimo Acquisition Corp. has accepted for payment all shares validly tendered and not validly withdrawn pursuant to its tender offer for all outstanding shares of common stock of GenMark Diagnostics, Inc. (NASDAQ: GNMK) at a price of USD
MoreBasel, 16 March 2021- Roche (SIX: RO, ROG; OTCQX: RHHBY) today announced the launch of the cobas® SARS-CoV-2 Variant Set 1 Test to detect and differentiate mutations found in variants that originated in the UK (B.1.1.7), South Africa (B.1.351), and Brazil (P.1). This research use only laboratory test can be used to help scientists track mutation
MoreRoche (SIX: RO, ROG; OTCQX: RHHBY) today announced that the New England Journal of Medicine has published Evrysdi™ (risdiplam) data from the dose finding Part 1 of the pivotal FIREFISH study in infants with symptomatic Type 1 spinal muscular atrophy (SMA).
MoreThe SARS-CoV-2 Rapid Antigen Test Nasal uses a nasal swab to quickly and conveniently collect specimens from people suspected of having an active infection
MoreRoche (SIX: RO, ROG; OTCQX: RHHBY) today confirmed that the U.S. Department of Health and Human Services (HHS) and the Department of Defense (DOD) will purchase additional supply of Regeneron's casirivimab and imdevimab antibody cocktail for use in non-hospitalised COVID-19 patients as part of Operation Warp Speed.
MoreBasel, 11 December 2020 - Roche (SIX: RO, ROG; OTCQX: RHHBY) announced today that it has launched a high-throughput SARS-CoV-2 antigen test as an aid in the diagnosis of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infections, in markets accepting the CE Mark. Roche has also filed for Emergency Use Authorisation (EUA) from the U.S.
Moretoday announced a partnership with Moderna Inc. to utilise the Elecsys® Anti-SARS-CoV-2 S antibody test in Moderna's mRNA-1273 vaccine research trials. This will facilitate the quantitative measurement of SARS-CoV-2 antibodies and help to establish a correlation between vaccine-induced protection and levels of anti-receptor binding domain (RBD) antibodies.
MoreThe ranking acknowledges Roche's commitment to sustainability as an integral part of its business strategy
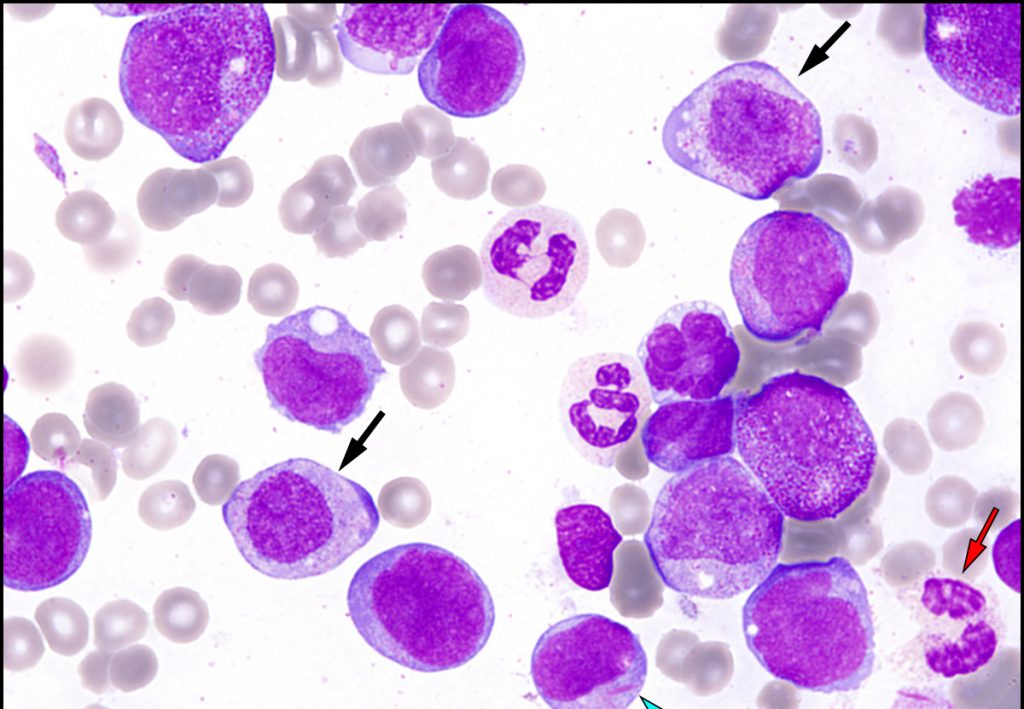
MoreApproval supported by data from phase III confirmatory trials, VIALE-A and VIALE-C
VIALE-A study showed Venclexta plus azacitidine significantly improved overall survival in newly diagnosed AML compared to azacitidine alone
Supplemental New Drug Applications approved under the FDA's Real-Time Oncology Review pilot programme and Project Orbis initiative