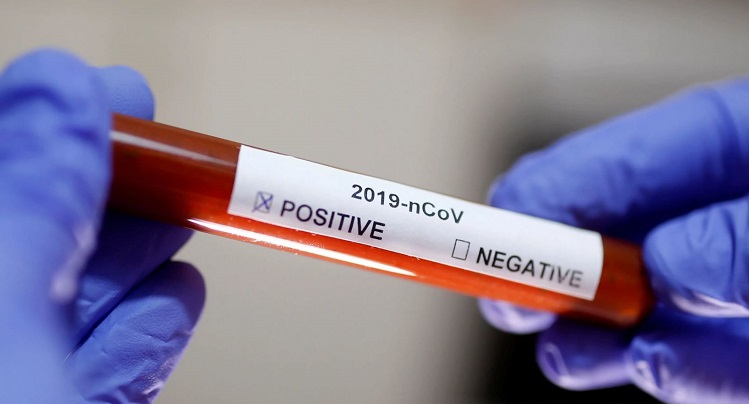
Basel, 16 March 2021- Roche (SIX: RO, ROG; OTCQX: RHHBY) today announced the launch of the cobas® SARS-CoV-2 Variant Set 1 Test to detect and differentiate mutations found in variants that originated in the UK (B.1.1.7), South Africa (B.1.351), and Brazil (P.1). This research use only laboratory test can be used to help scientists track mutation
MoreThe U.S. Food and Drug Administration formally authorized the use of the Johnson & Johnson’s one-dose vaccine Saturday, clearing the way for shots to go into arms as early as Monday.
MoreA North Carolina sport supplement company owner was sentenced to one year and one day in federal prison after pleading guilty to introducing unapproved new drugs into interstate commerce, the Department of Justice announced.
MoreThe Food and Drug Administration recently granted emergency use authorization to a coronavirus vaccine from Pfizer, and approval for Moderna’s version is anticipated shortly.
MoreAs U.S. hospitals ramped up COVID-19 vaccinations Tuesday with the first vaccine approved for emergency use, federal regulators issued a favorable review of a second vaccine needed to combat the country's worsening crisis.
MorePrimary efficacy analysis of the Phase 3 COVE study of mRNA-1273 involving 30,000 participants included 196 cases of COVID-19, of which 30 cases were severe
MoreXofluza is the first single-dose influenza medicine approved to prevent influenza for those who have had contact with an infected person (post-exposure prophylaxis)
MoreThe United States filed suit to halt the sale by a New Jersey entity of an unapproved “nano silver” product previously touted as a COVID-19 treatment, the Department of Justice announced today.
More